Therapeutic Potential of Jasmonic Acid and Its Derivatives
Abstract
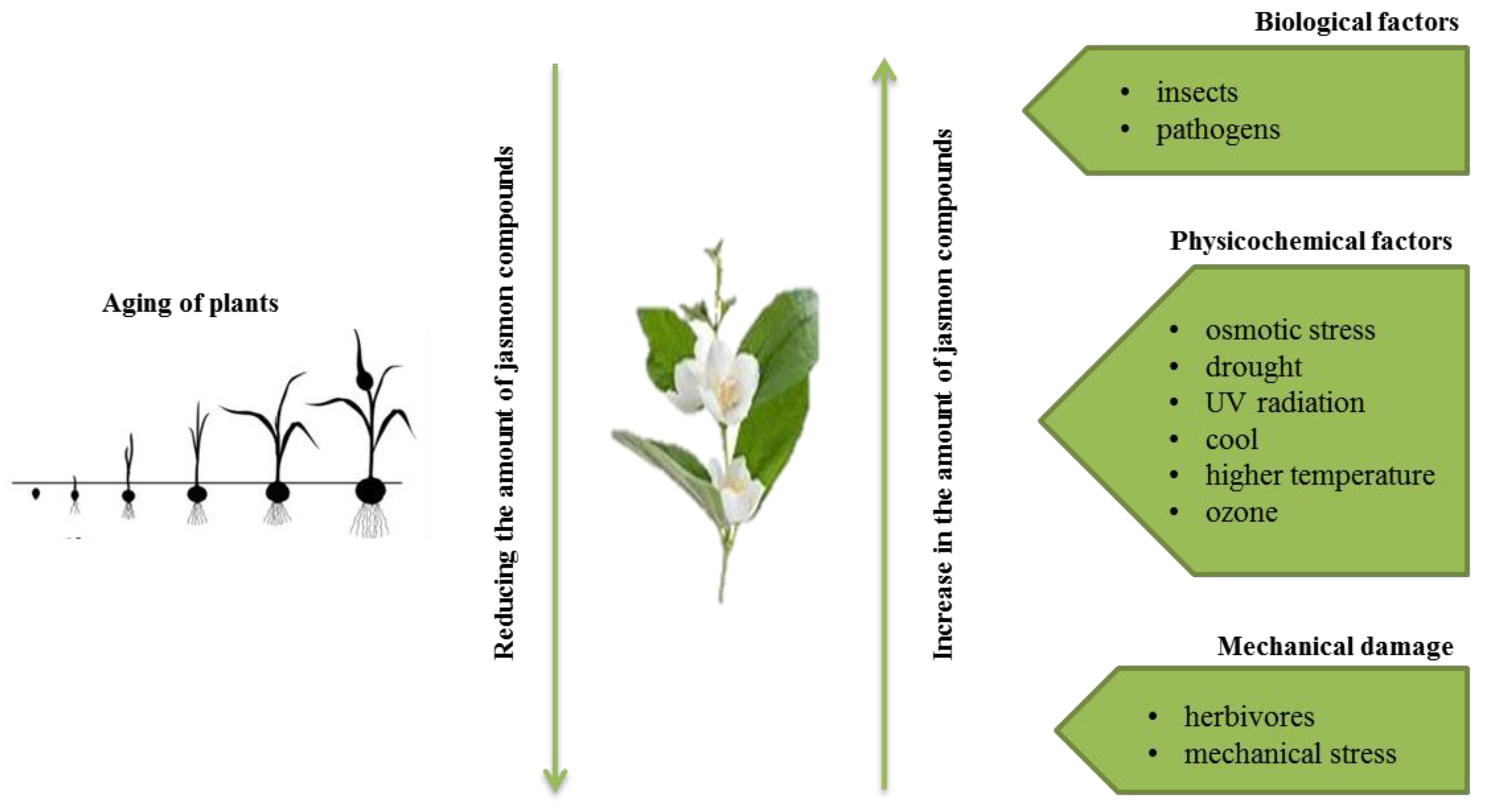
:1. Jasmonate Compounds in Plants
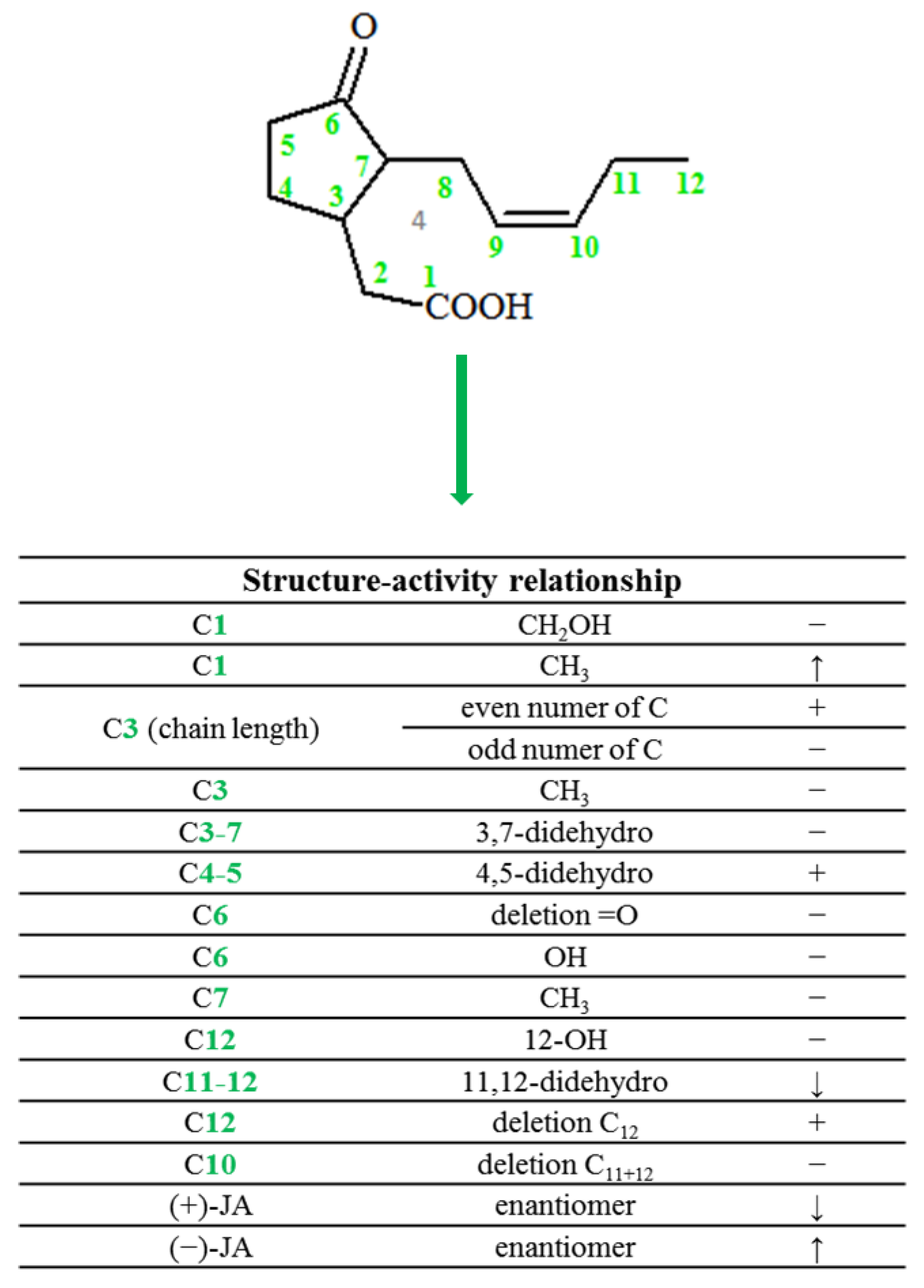
2. Chemical Structure of Jasmonate
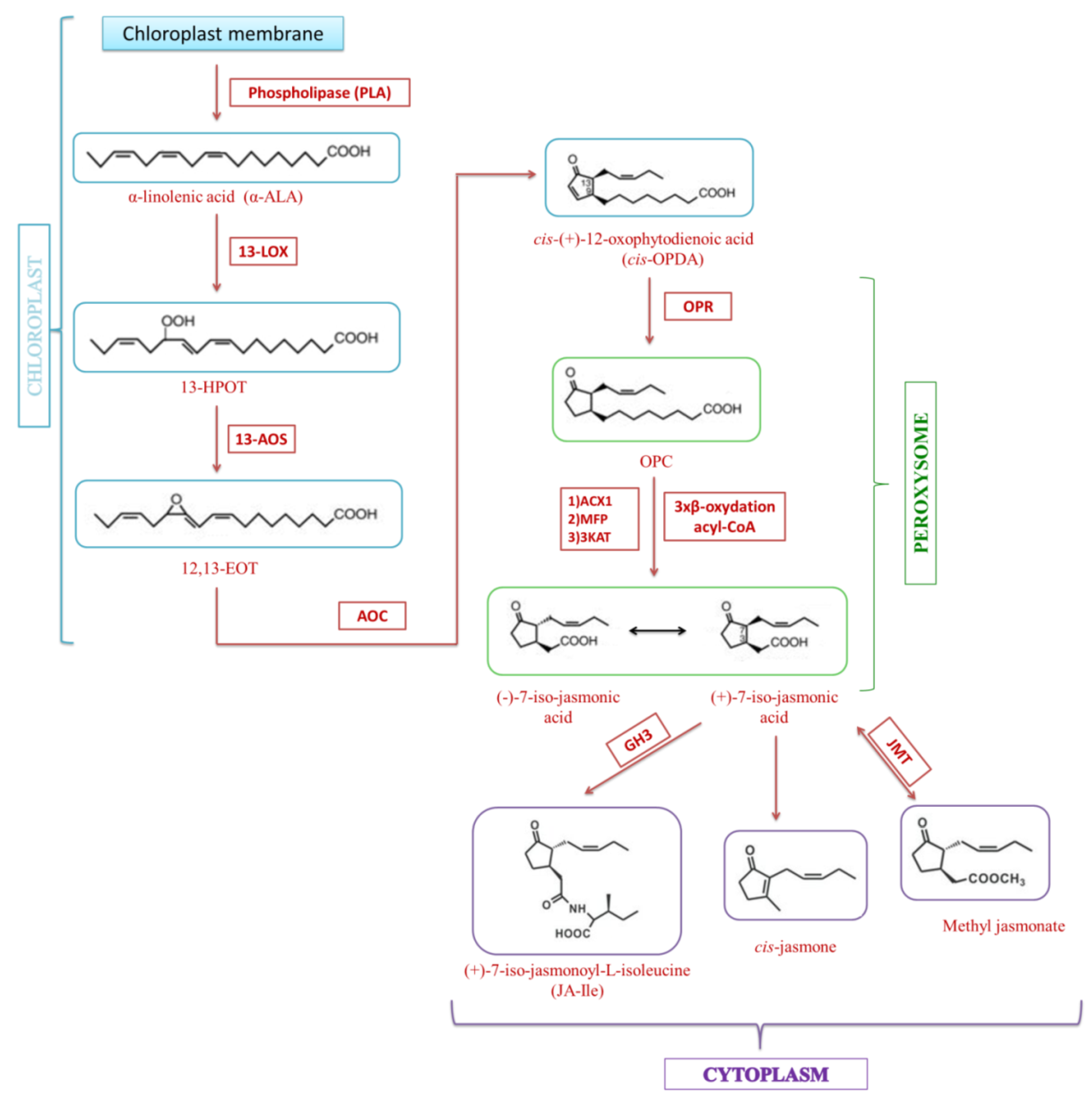
3. Jasmonate Biosynthesis
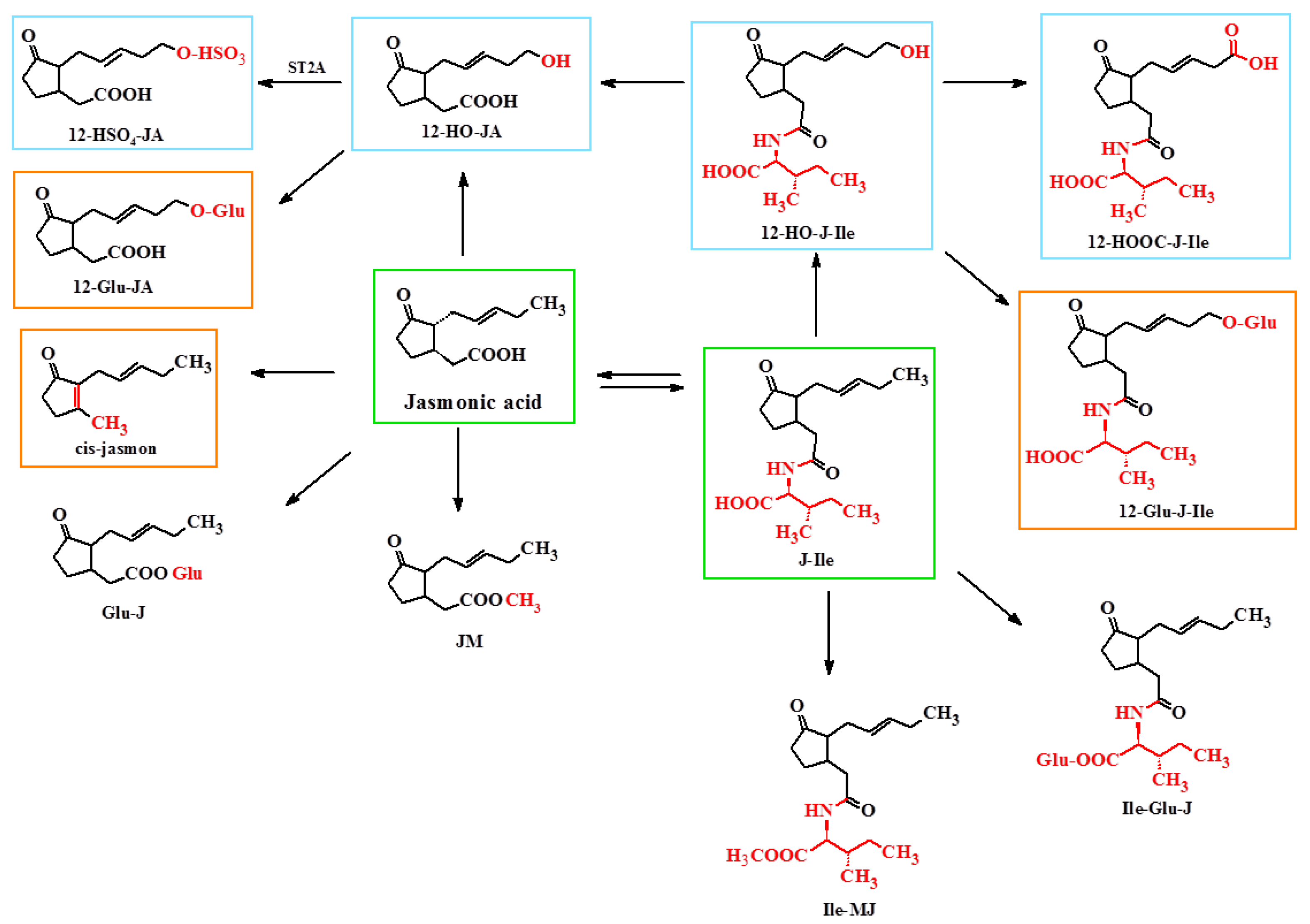
4. Metabolism of Jasmonate
5. The Role of Jasmonates in Plant Growth
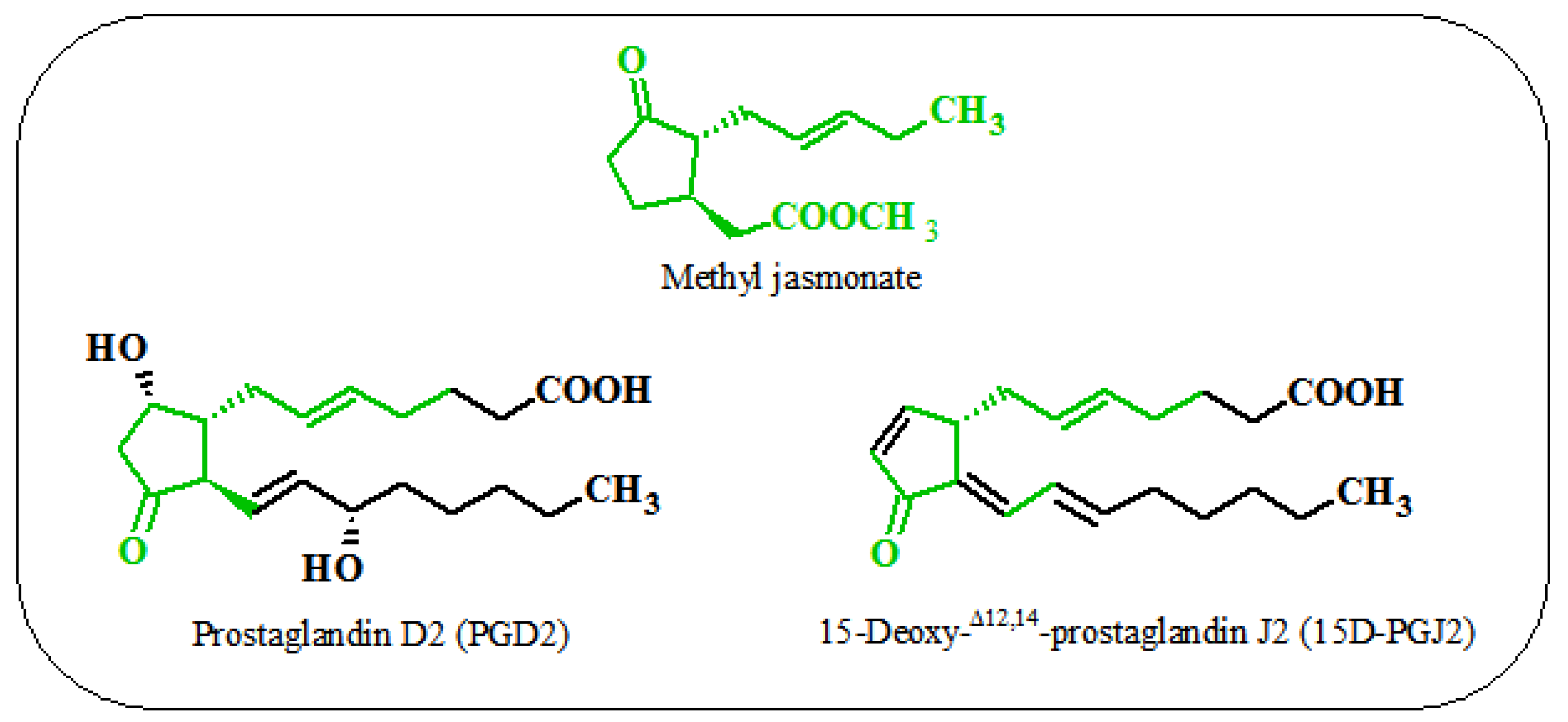
6. Similarities between the Action of Jasmonates on Plant and Animal Cells
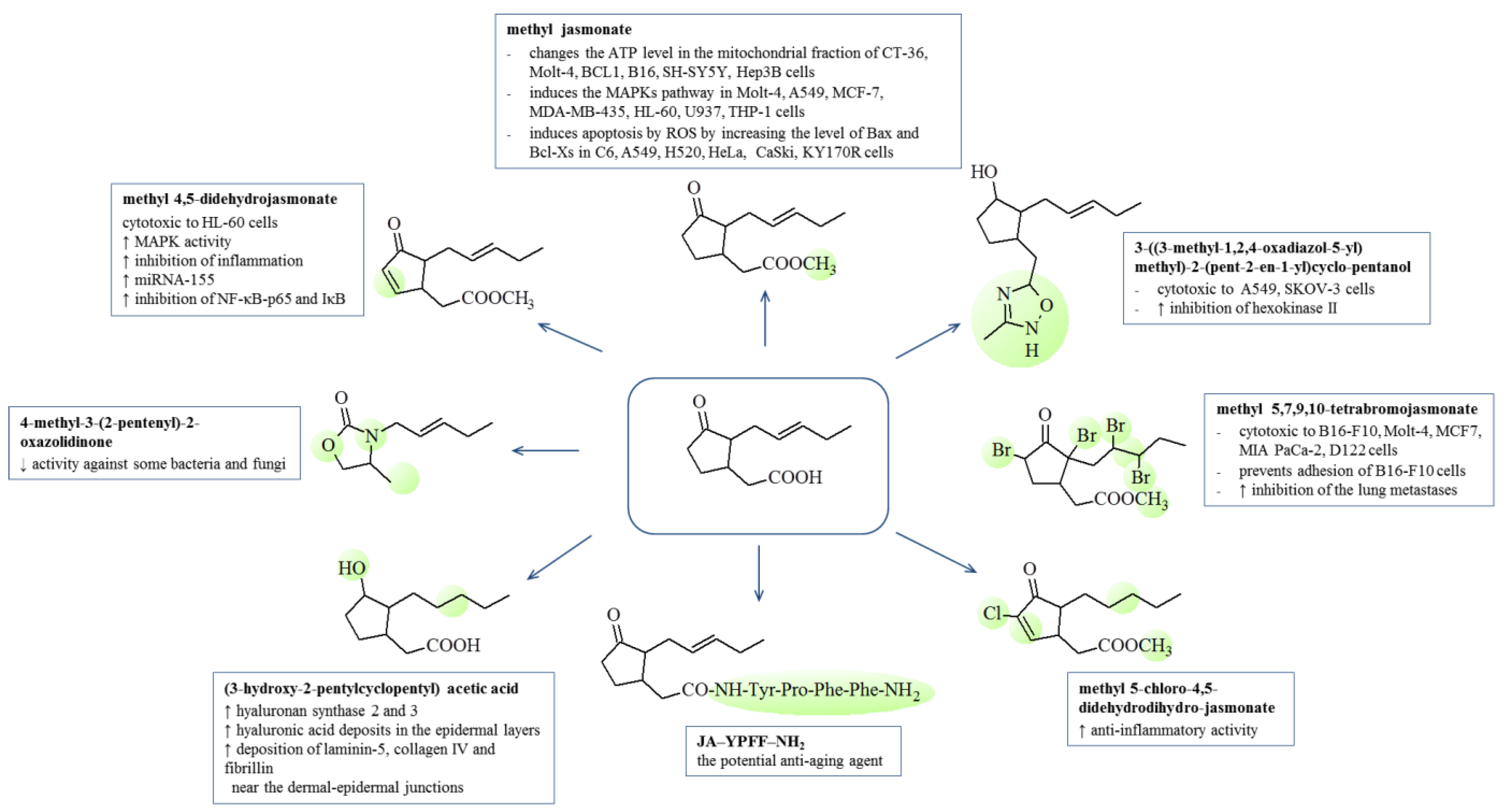
7. Biological Activity of Jasmonates and Their Derivatives
7.1. Anti-Inflammatory
7.2. Anticancer
7.3. Cosmetic Activities
8. In Vivo Effect of Methyl Jasmonate and Synthetic Tetrabromojasmonate
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saniewski, M. The Role of Jasmonates in Ethylene Biosynthesis. In Biology and Biotechnology of the Plant Hormone Ethylene; Kanellis, A.K., Chang, C., Kende, H., Grierson, D., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1997; pp. 39–45. ISBN 978-94-011-5546-5. [Google Scholar]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl Jasmonate Induced Oxidative Stress and Accumulation of Secondary Metabolites in Plant Cell and Organ Cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef] [Green Version]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrín-Novo, J.V.; Alves, A.; et al. Production of Toxic Metabolites by Two Strains of Lasiodiplodia Theobromae, Isolated from a Coconut Tree and a Human Patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef]
- Demole, E.; Lederer, E.; Mercier, D. Isolement et Détermination de La Structure Du Jasmonate de Méthyle, Constituant Odorant Caractéristique de l’essence de Jasmin. Helv. Chim. Acta 1962, 45, 675–685. [Google Scholar] [CrossRef]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.D.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl Jasmonate: Putative Mechanisms of Action on Cancer Cells Cycle, Metabolism, and Apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef] [PubMed]
- Wilmowicz, E.; Frankowski, K.; Sidłowska, M.; Kućko, A.; Kesy, J.; Gasiorowski, A.; Glazińska, P.; Kopcewicz, J. Jasmonate biosynthesis--the latest discoveries. Postepy Biochem. 2012, 58, 26–33. [Google Scholar] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi Pirbalouti, A.; Sajjadi, S.E.; Parang, K. A Review (Research and Patents) on Jasmonic Acid and Its Derivatives. Arch. Pharm. 2014, 347, 229–239. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Kombrink, E. Jasmonates: Structural Requirements for Lipid-Derived Signals Active in Plant Stress Responses and Development. ACS Chem. Biol. 2010, 5, 63–77. [Google Scholar] [CrossRef]
- Han, Y.; Bai, Y.; Xiao, Y.; Du, F.; Liang, Y.; Tan, Z.; Zhao, M.; Liu, H. Simultaneous Discrimination of Jasmonic Acid Stereoisomers by CE-QTOF-MS Employing the Partial Filling Technique. Electrophoresis 2011, 32, 2693–2699. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Jez, J. Structural Biology of Jasmonic Acid Metabolism and Responses in Plants. In Plant Structural Biology: Hormonal Regulations; Springer: Berlin/Heidelberg, Germany, 2018; pp. 67–82. ISBN 978-3-319-91351-3. [Google Scholar]
- Koda, Y.; Kikuta, Y.; Tazaki, H.; Tsujino, Y.; Sakamura, S.; Yoshihara, T. Potato Tuber-Inducing Activities of Jasmonic Acid and Related Compounds. Phytochemistry 1991, 30, 1435–1438. [Google Scholar] [CrossRef]
- Krumm, T.; Bandemer, K.; Boland, W. Induction of Volatile Biosynthesis in the Lima Bean (Phaseolus Lunatus) by Leucine- and Isoleucine Conjugates of 1-Oxo- and 1-Hydroxyindan-4-Carboxylic Acid: Evidence for Amino Acid Conjugates of Jasmonic Acid as Intermediates in the Octadecanoid Signalling Pathway. FEBS Lett. 1995, 377, 523–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flescher, E. Jasmonates in Cancer Therapy. Cancer Lett. 2007, 245, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, Metabolism, and Signaling by Proteins Activating and Repressing Transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- León, J.; Sánchez-Serrano, J.J. Molecular Biology of Jasmonic Acid Biosynthesis in Plants. Plant Physiol. Biochem. 1999, 37, 373–380. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of Jasmonic Acid in Plants: The Molecular Point of View. Plant Cell Rep. 2021, 5, 1–24. [Google Scholar] [CrossRef]
- Larrieu, A.; Vernoux, T. Q&A: How Does Jasmonate Signaling Enable Plants to Adapt and Survive? BMC Biol. 2016, 14, 79. [Google Scholar] [CrossRef]
- Cheong, J.-J.; Choi, Y.D. Methyl Jasmonate as a Vital Substance in Plants. Trends Genet. TIG 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Enomoto, H.; Miyamoto, K. Unique Localization of Jasmonic Acid-Related Compounds in Developing Phaseolus Vulgaris, L. (Common Bean) Seeds Revealed through Desorption Electrospray Ionization-Mass Spectrometry Imaging. Phytochemistry 2021, 188, 112812. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonate Signaling in Plant Stress Responses and Development–Active and Inactive Compounds. New Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef]
- Wasternack, C. How Jasmonates Earned Their Laurels: Past and Present. J. Plant Growth Regul. 2015, 34, 761–794. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, H.; Li, L.; Zhai, Q.; Qi, L.; Zhou, W.; Liu, X.; Li, H.; Zheng, W.; Sun, J.; et al. The Arabidopsis Mediator Subunit MED25 Differentially Regulates Jasmonate and Abscisic Acid Signaling through Interacting with the MYC2 and ABI5 Transcription Factors. Plant Cell 2012, 24, 2898–2916. [Google Scholar] [CrossRef] [Green Version]
- Staswick, P.E.; Tiryaki, I. The Oxylipin Signal Jasmonic Acid Is Activated by an Enzyme That Conjugates It to Isoleucine in Arabidopsis. Plant Cell 2004, 16, 2117–2127. [Google Scholar] [CrossRef] [Green Version]
- Suza, W.P.; Staswick, P.E. The Role of JAR1 in Jasmonoyl-L-Isoleucine Production during Arabidopsis Wound Response. Planta 2008, 227, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Swiatek, A.; Azmi, A.; Stals, H.; Inzé, D.; Van Onckelen, H. Jasmonic Acid Prevents the Accumulation of Cyclin B1;1 and CDK-B in Synchronized Tobacco BY-2 Cells. FEBS Lett. 2004, 572, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, T.; Bandemer, K.; Boland, W. Biosynthesis of Cis-Jasmone: A Pathway for the Inactivation and the Disposal of the Plant Stress Hormone Jasmonic Acid to the Gas Phase? Helv. Chim. Acta 1997, 80, 838–850. [Google Scholar] [CrossRef]
- Gidda, S.K.; Miersch, O.; Levitin, A.; Schmidt, J.; Wasternack, C.; Varin, L. Biochemical and Molecular Characterization of a Hydroxyjasmonate Sulfotransferase from Arabidopsis Thaliana. J. Biol. Chem. 2003, 278, 17895–17900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaller, F.; Schaller, A.; Stintzi, A. Biosynthesis and Metabolism of Jasmonates. J. Plant Growth Regul. 2004, 23, 179–199. [Google Scholar] [CrossRef]
- Piotrowska, A.; Bajguz, A. Conjugates of Abscisic Acid, Brassinosteroids, Ethylene, Gibberellins, and Jasmonates. Phytochemistry 2011, 72, 2097–2112. [Google Scholar] [CrossRef]
- Antognoni, F.; Faudale, M.; Poli, F.; Biondi, S. Methyl Jasmonate Differentially Affects Tocopherol Content and Tyrosine Amino Transferase Activity in Cultured Cells of Amaranthus Caudatus and Chenopodium Quinoa. Plant Biol. 2009, 11, 161–169. [Google Scholar] [CrossRef]
- Luo, H.; He, W.; Li, D.; Bao, Y.; Riaz, A.; Xiao, Y.; Song, J.; Liu, C. Effect of Methyl Jasmonate on Carotenoids Biosynthesis in Germinated Maize Kernels. Food Chem. 2020, 307, 125525. [Google Scholar] [CrossRef] [PubMed]
- Saniewski, M.; Czapski, J. The Effect of Methyl Jasmonate on Lycopene and β-Carotene Accumulation in Ripening Red Tomatoes. Experientia 1983, 39, 1373–1374. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Wiczkowski, W.; Koczkodaj, D.; Mitrus, J. The Effect of Methyl Jasmonate on Accumulation of 2-Phenylethylamine and Putrescine in Seedlings of Common Buckwheat (Fagopyrum Esculentum). Acta Physiol. Plant. 2011, 33, 897–903. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zeng, H.; Cai, Q.; Zhou, X.; Yin, C. Exogenous Jasmonic Acid and Cytokinin Antagonistically Regulate Rice Flag Leaf Senescence by Mediating Chlorophyll Degradation, Membrane Deterioration, and Senescence-Associated Genes Expression. J. Plant Growth Regul. 2016, 35, 366–376. [Google Scholar] [CrossRef]
- Staswick, P.E.; Huang, J.-F.; Rhee, Y. Nitrogen and Methyl Jasmonate Induction of Soybean Vegetative Storage Protein Genes 1. Plant Physiol. 1991, 96, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Rohwer, C.L.; Erwin, J.E. Horticultural Applications of Jasmonates: A Review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Jasmonates: Mechanisms and Functions in Abiotic Stress Tolerance of Plants. Biocatal. Agric. Biotechnol. 2019, 20, 101210. [Google Scholar] [CrossRef]
- Maslenkova, L.T.; Miteva, T.S.; Popova, L.P. Changes in the Polypeptide Patterns of Barley Seedlings Exposed to Jasmonic Acid and Salinity. Plant Physiol. 1992, 98, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León-Morcillo, R.J.; Ángel, J.; Martín, R.; Vierheilig, H.; Ocampo, J.A.; García-Garrido, J.M. Late Activation of the 9-Oxylipin Pathway during Arbuscular Mycorrhiza Formation in Tomato and Its Regulation by Jasmonate Signalling. J. Exp. Bot. 2012, 63, 3545–3558. [Google Scholar] [CrossRef]
- Hause, B.; Mrosk, C.; Isayenkov, S.; Strack, D. Jasmonates in Arbuscular Mycorrhizal Interactions. Phytochemistry 2007, 68, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, Z.; Li, X. Effect of Methyl Jasmonate on Cadmium Uptake and Antioxidative Capacity in Kandelia Obovata Seedlings under Cadmium Stress. Ecotoxicol. Environ. Saf. 2014, 104, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.; Müller, S.; Bauer, P. Suppression of Fe Deficiency Gene Expression by Jasmonate. Plant Physiol. Biochem. 2011, 49, 530–536. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Vardar, F. Effect of Methyl Jasmonate on In-Vitro Pollen Germination and Tube Elongation of Pinus Nigra. Protoplasma 2020, 257, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Muradoğlu, F.; Yıldız, K.; Balta, F. Methyl Jasmonate Influences of Pollen Germination and Pollen Tube Growth of Apricot (Prunus Armeniaca L.). YYU J. Agric. Sci. 2010, 20, 183–188. [Google Scholar]
- Schweizer, P.; Gees, R.; Mosinger, E. Effect of Jasmonic Acid on the Interaction of Barley (Hordeum Vulgare L.) with the Powdery Mildew Erysiphe Graminis f.Sp. Hordei. Plant Physiol. 1993, 102, 503–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziurka, K.; Dziurka, M.; Warchoł, M.; Czyczyło-Mysza, I.; Marcińska, I.; Noga, A.; Kapłoniak, K.; Skrzypek, E. Endogenous Phytohormone Profile during Oat (Avena Sativa L.) Haploid Embryo Development. Vitro Cell. Dev. Biol. Plant 2019, 55, 221–229. [Google Scholar] [CrossRef]
- Pigolev, A.; Miroshnichenko, D.; Dolgov, S.; Savchenko, T. Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum Aestivum L. Plants Basel Switz. 2021, 10, 219. [Google Scholar] [CrossRef]
- Corbineau, F.; Rudnicki, R.M.; Côme, D. The Effects of Methyl Jasmonate on Sunflower (Helianthus Annuus L.) Seed Germination and Seedling Development. Plant Growth Regul. 1988, 7, 157–169. [Google Scholar] [CrossRef]
- Andrade, A.; Escalante, M.; Vigliocco, A.; del Carmen Tordable, M.; Alemano, S. Involvement of Jasmonates in Responses of Sunflower (Helianthus Annuus) Seedlings to Moderate Water Stress. Plant Growth Regul. 2017, 83, 501–511. [Google Scholar] [CrossRef]
- Concha, C.M.; Figueroa, N.E.; Poblete, L.A.; Oñate, F.A.; Schwab, W.; Figueroa, C.R. Methyl Jasmonate Treatment Induces Changes in Fruit Ripening by Modifying the Expression of Several Ripening Genes in Fragaria Chiloensis Fruit. Plant Physiol. Biochem. 2013, 70, 433–444. [Google Scholar] [CrossRef]
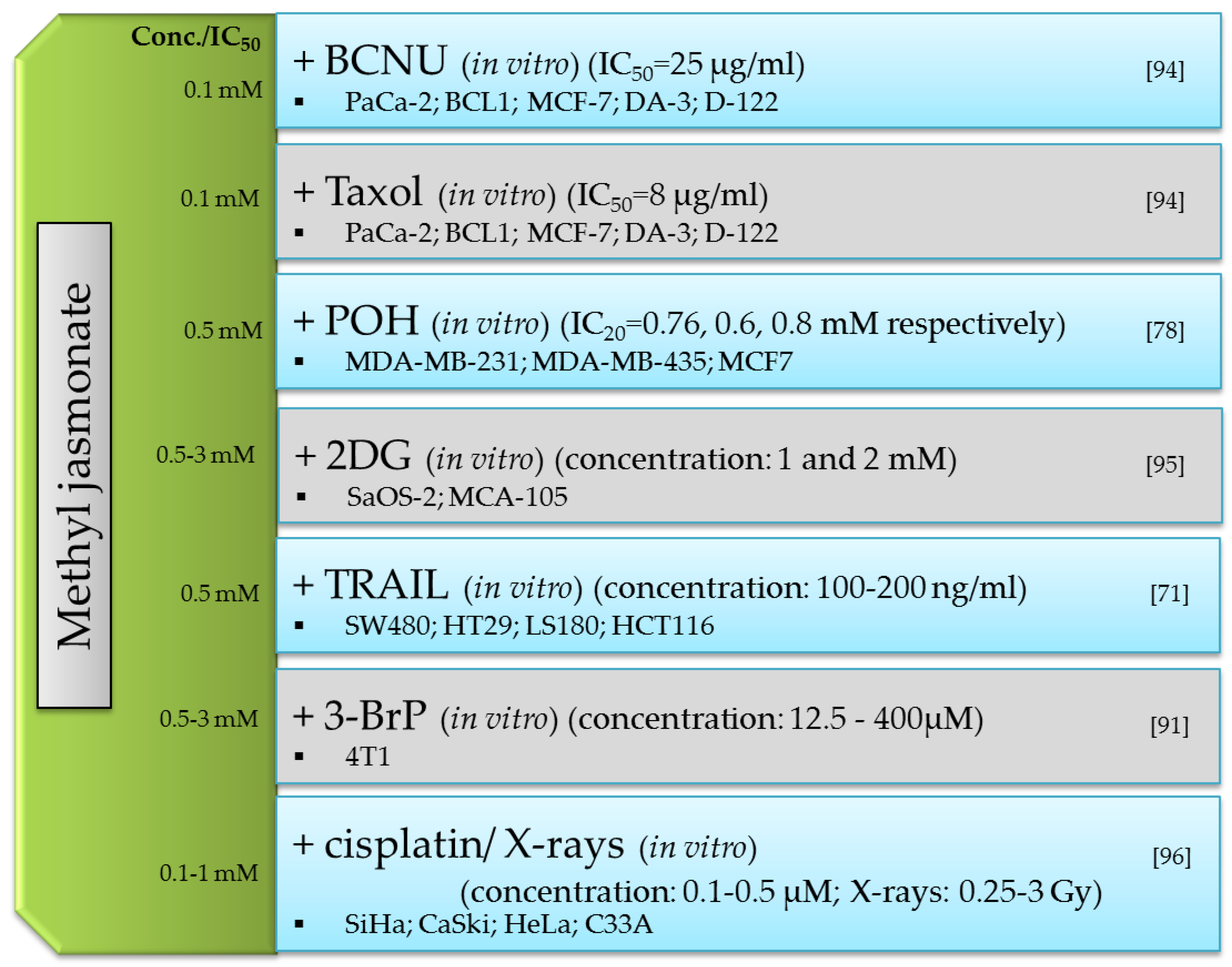
- Fang, C.; Zhang, H.; Wan, J.; Wu, Y.; Li, K.; Jin, C.; Chen, W.; Wang, S.; Wang, W.; Zhang, H.; et al. Control of Leaf Senescence by an MeOH-Jasmonates Cascade That Is Epigenetically Regulated by OsSRT1 in Rice. Mol. Plant 2016, 9, 1366–1378. [Google Scholar] [CrossRef] [Green Version]
- ISHIHARA, A.; OGURA, Y.; TEBAYASHI, S.; IWAMURA, H. Jasmonate-Induced Changes in Flavonoid Metabolism in Barley (Hordeum Vulgare) Leaves. Biosci. Biotechnol. Biochem. 2002, 66, 2176–2182. [Google Scholar] [CrossRef] [Green Version]
- Shan, X.; Wang, J.; Chua, L.; Jiang, D.; Peng, W.; Xie, D. The Role of Arabidopsis Rubisco Activase in Jasmonate-Induced Leaf Senescence. Plant Physiol. 2011, 155, 751–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Ma, H. Male Fertility: A Case of Enzyme Identity. Curr. Biol. 2000, 10, R904–R907. [Google Scholar] [CrossRef] [Green Version]
- Ruduś, I.; Weiler, E.W.; Kępczyńska, E. Do Stress-Related Phytohormones, Abscisic Acid and Jasmonic Acid Play a Role in the Regulation of Medicago Sativa L. Somatic Embryogenesis? Plant Growth Regul. 2009, 59, 159–169. [Google Scholar] [CrossRef]
- Raya-González, J.; Pelagio-Flores, R.; López-Bucio, J. The Jasmonate Receptor COI1 Plays a Role in Jasmonate-Induced Lateral Root Formation and Lateral Root Positioning in Arabidopsis Thaliana. J. Plant Physiol. 2012, 169, 1348–1358. [Google Scholar] [CrossRef]
- Asai, T.; Stone, J.M.; Heard, J.E.; Kovtun, Y.; Yorgey, P.; Sheen, J.; Ausubel, F.M. Fumonisin B1-Induced Cell Death in Arabidopsis Protoplasts Requires Jasmonate-, Ethylene-, and Salicylate-Dependent Signaling Pathways. Plant Cell 2000, 12, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naill, M.C.; Roberts, S.C. Cell Cycle Analysis of Taxus Suspension Cultures at the Single Cell Level as an Indicator of Culture Heterogeneity. Biotechnol. Bioeng. 2005, 90, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Kiyota, H.; Sakai, S.; Honma, Y. Induction of Differentiation of Human Myeloid Leukemia Cells by Jasmonates, Plant Hormones. Leukemia 2004, 18, 1413–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danon, A.; Miersch, O.; Felix, G.; Camp, R.G.L.; Apel, K. Concurrent Activation of Cell Death-Regulating Signaling Pathways by Singlet Oxygen in Arabidopsis Thaliana. Plant J. Cell Mol. Biol. 2005, 41, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Conti, M. Cyclopentenone: A Special Moiety for Anticancer Drug Design. Anticancer Drugs 2006, 17, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.T.; Lee, H.J.; Yoo, E.S.; Hong, J.; Bao, B.; Choi, J.S.; Jung, J.H. New Jasmonate Analogues as Potential Anti-Inflammatory Agents. Bioorg. Med. Chem. 2008, 16, 10228–10235. [Google Scholar] [CrossRef] [PubMed]
- Jarocka-Karpowicz, I.; Markowska, A. Jasmonate Compounds and Their Derivatives in the Regulation of the Neoplastic Processes. Molecules 2021, 26, 2901. [Google Scholar] [CrossRef]
- Dang, H.T.; Lee, Y.M.; Kang, G.J.; Yoo, E.S.; Hong, J.; Lee, S.M.; Lee, S.K.; Pyee, Y.; Chung, H.-J.; Moon, H.R.; et al. In Vitro Stability and in Vivo Anti-Inflammatory Efficacy of Synthetic Jasmonates. Bioorg. Med. Chem. 2012, 20, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Maeng, K.; Dang, H.-T.; Kang, G.-J.; Ryou, C.; Jung, J.H.; Kang, H.-K.; Prchal, J.T.; Yoo, E.-S.; Yoon, D. Anti-Inflammatory Effect of Methyl Dehydrojasmonate (J2) Is Mediated by the NF-ΚB Pathway. J. Mol. Med. Berl. Ger. 2011, 89, 83–90. [Google Scholar] [CrossRef]
- Reischer, D.; Heyfets, A.; Shimony, S.; Nordenberg, J.; Kashman, Y.; Flescher, E. Effects of Natural and Novel Synthetic Jasmonates in Experimental Metastatic Melanoma. Br. J. Pharmacol. 2007, 150, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Sucu, B.O.; Ipek, O.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of Novel Methyl Jasmonate Derivatives and Evaluation of Their Biological Activity in Various Cancer Cell Lines. Bioorganic Chem. 2019, 91, 103146. [Google Scholar] [CrossRef]
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The Anti-Cancer Activities of Jasmonates. Cancer Chemother. Pharmacol. 2013, 71, 275–285. [Google Scholar] [CrossRef]
- Fingrut, O.; Reischer, D.; Rotem, R.; Goldin, N.; Altboum, I.; Zan-Bar, I.; Flescher, E. Jasmonates Induce Nonapoptotic Death in High-Resistance Mutant P53-Expressing B-Lymphoma Cells. Br. J. Pharmacol. 2005, 146, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Goldin, N.; Arzoine, L.; Heyfets, A.; Israelson, A.; Zaslavsky, Z.; Bravman, T.; Bronner, V.; Notcovich, A.; Shoshan-Barmatz, V.; Flescher, E. Methyl Jasmonate Binds to and Detaches Mitochondria-Bound Hexokinase. Oncogene 2008, 27, 4636–4643. [Google Scholar] [CrossRef] [Green Version]
- Tong, Q.-S.; Jiang, G.-S.; Zheng, L.-D.; Tang, S.-T.; Cai, J.-B.; Liu, Y.; Zeng, F.-Q.; Dong, J.-H. Methyl Jasmonate Downregulates Expression of Proliferating Cell Nuclear Antigen and Induces Apoptosis in Human Neuroblastoma Cell Lines. Anticancer. Drugs 2008, 19, 573–581. [Google Scholar] [CrossRef]
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel Anticancer Agents Acting Directly and Selectively on Human Cancer Cell Mitochondria. Cancer Res. 2005, 65, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.Y.; Oh, S.Y.; Han, S.I.; Park, H.G.; Yoo, M.-A.; Kang, H.S. Methyl Jasmonate Induces Apoptosis through Induction of Bax/Bcl-XS and Activation of Caspase-3 via ROS Production in A549 Cells. Oncol. Rep. 2004, 12, 1233–1238. [Google Scholar] [CrossRef]
- Yeruva, L.; Elegbede, J.A.; Carper, S.W. Methyl Jasmonate Decreases Membrane Fluidity and Induces Apoptosis through Tumor Necrosis Factor Receptor 1 in Breast Cancer Cells. Anticancer. Drugs 2008, 19, 766–776. [Google Scholar] [CrossRef] [Green Version]
- Yeruva, L.; Hall, C.; Elegbede, J.A.; Carper, S.W. Perillyl Alcohol and Methyl Jasmonate Sensitize Cancer Cells to Cisplatin. Anticancer. Drugs 2010, 21, 1–9. [Google Scholar] [CrossRef]
- Cohen, S.; Flescher, E. Methyl Jasmonate: A Plant Stress Hormone as an Anti-Cancer Drug. Phytochemistry 2009, 70, 1600–1609. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.H.; Park, M.J.; Kim, S.M.; Yoon, C.S.; Joo, Y.M.; Park, J.S.; Han, S.I.; Park, H.G.; Kang, H.S. Induction of Heat Shock Protein 72 in C6 Glioma Cells by Methyl Jasmonate through ROS-Dependent Heat Shock Factor 1 Activation. Int. J. Mol. Med. 2005, 16, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kniazhanski, T.; Jackman, A.; Heyfets, A.; Gonen, P.; Flescher, E.; Sherman, L. Methyl Jasmonate Induces Cell Death with Mixed Characteristics of Apoptosis and Necrosis in Cervical Cancer Cells. Cancer Lett. 2008, 271, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhao, J.; Xiao, X.; Tao, D.; Gu, C.; Tong, Q.; Luo, B.; Wang, L.; Zeng, F. AN N-Terminal Smac Peptide Sensitizes Human Prostate Carcinoma Cells to Methyl Jasmonate-Induced Apoptosis. Cancer Lett. 2011, 302, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, E.; Pedersen, P.L. High Aerobic Glycolysis of Rat Hepatoma Cells in Culture: Role of Mitochondrial Hexokinase. Proc. Natl. Acad. Sci. USA 1977, 74, 3735–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Chen, K.; Wang, F.; Dai, W.; Li, S.; Feng, J.; Wu, L.; Liu, T.; Xu, S.; Xia, Y.; et al. Methyl Jasmonate Leads to Necrosis and Apoptosis in Hepatocellular Carcinoma Cells via Inhibition of Glycolysis and Represses Tumor Growth Mice. Oncotarget 2017, 8, 45965–45980. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Marquez, M.; Galano, A. The Reactions of Plant Hormones with Reactive Oxygen Species: Chemical Insights at a Molecular Level. J. Mol. Model. 2018, 24, 255. [Google Scholar] [CrossRef]
- Besson, J.C.F.; de Carvalho Picoli, C.; Matioli, G.; Natali, M.R.M. Methyl Jasmonate: A Phytohormone with Potential for the Treatment of Inflammatory Bowel Diseases. J. Pharm. Pharmacol. 2018, 70, 178–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezekwudo, D.; Shashidharamurthy, R.; Devineni, D.; Bozeman, E.; Palaniappan, R.; Selvaraj, P. Inhibition of Expression of Anti-Apoptotic Protein Bcl-2 and Induction of Cell Death in Radioresistant Human Prostate Adenocarcinoma Cell Line (PC-3) by Methyl Jasmonate. Cancer Lett. 2008, 270, 277–285. [Google Scholar] [CrossRef]
- Zhang, M.; Su, L.; Xiao, Z.; Liu, X.; Liu, X. Methyl Jasmonate Induces Apoptosis and Pro-Apoptotic Autophagy via the ROS Pathway in Human Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 187–199. [Google Scholar]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants Basel Switz. 2021, 10, 569. [Google Scholar] [CrossRef]
- Yousefi, S.; Darvishi, P.; Yousefi, Z.; Pourfathollah, A.A. Effect of Methyl Jasmonate and 3-Bromopyruvate Combination Therapy on Mice Bearing the 4 T1 Breast Cancer Cell Line. J. Bioenerg. Biomembr. 2020, 52, 103–111. [Google Scholar] [CrossRef]
- Cai, S.; Xu, Y.; Cooper, R.J.; Ferkowicz, M.J.; Hartwell, J.R.; Pollok, K.E.; Kelley, M.R. Mitochondrial Targeting of Human O6-Methylguanine DNA Methyltransferase Protects against Cell Killing by Chemotherapeutic Alkylating Agents. Cancer Res. 2005, 65, 3319–3327. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tournier, C. Regulation of Cellular Functions by the ERK5 Signalling Pathway. Cell. Signal. 2006, 18, 753–760. [Google Scholar] [CrossRef]
- Heyfets, A.; Flescher, E. Cooperative Cytotoxicity of Methyl Jasmonate with Anti-Cancer Drugs and 2-Deoxy-D-Glucose. Cancer Lett. 2007, 250, 300–310. [Google Scholar] [CrossRef]
- Elia, U.; Flescher, E. PI3K/Akt Pathway Activation Attenuates the Cytotoxic Effect of Methyl Jasmonate toward Sarcoma Cells. Neoplasia N. Y. 2008, 10, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- Milrot, E.; Jackman, A.; Flescher, E.; Gonen, P.; Kelson, I.; Keisari, Y.; Sherman, L. Enhanced Killing of Cervical Cancer Cells by Combinations of Methyl Jasmonate with Cisplatin, X or Alpha Radiation. Invest. New Drugs 2013, 31, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Kapuścińska, A.; Nowak, I. Wykorzystanie Wybranych Fitohormonów w Przemyśle Kosmetycznym i Farmaceutycznym; Rośliny–przegląd Wybranych Zagadnień: Lublin, Poland, 2016; pp. 160–174. ISBN 978-83-65598-13-4. [Google Scholar]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Methyl Jasmonate. Food Chem. Toxicol. 2012, 50, S572–S576. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Zaprutko, L. Microwave Assisted Synthesis of Unsaturated Jasmone Heterocyclic Analogues as New Fragrant Substances. Eur. J. Med. Chem. 2009, 44, 3032–3039. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Michalak, J.; Kędzia, B.; Zaprutko, L. Ocena Aktywności Antybiotycznej Z-Jasmonu Oraz Jego Pochodnych Heterocyklicznych. Postępy Fitoter. 2017, 18, 171–177. [Google Scholar] [CrossRef]
- Safety Evaluation of Certain Food Additives: Prepared by the Eighty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: https://www.who.int/publications-detail-redirect/9789240004580 (accessed on 30 July 2021).
- Fingrut, O.; Flescher, E. Plant Stress Hormones Suppress the Proliferation and Induce Apoptosis in Human Cancer Cells. Leukemia 2002, 16, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Pereira Lopes, J.E.F.; Barbosa, M.R.; Stella, C.N.; Santos, W.A.; Pereira, E.M.; Nogueira-Neto, J.; Augusto, E.M.; Silva, L.V.; Smaili, S.S.; Gomes, L.F. In Vivo Anti-Angiogenic Effects Further Support the Promise of the Antineoplasic Activity of Methyl Jasmonate. Braz. J. Biol. Rev. Brasleira Biol. 2010, 70, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Palmieri, B.; Iannitti, T.; Capone, S.; Flescher, E. A Preliminary Study of the Local Treatment of Preneoplastic and Malignant Skin Lesions Using Methyl Jasmonate. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 333–336. [Google Scholar] [PubMed]
- Klippel, S.; Jakubikova, J.; Delmore, J.; Ooi, M.; McMillin, D.; Kastritis, E.; Laubach, J.; Richardson, P.G.; Anderson, K.C.; Mitsiades, C.S. Methyljasmonate Displays in Vitro and in Vivo Activity against Multiple Myeloma Cells. Br. J. Haematol. 2012, 159, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Gunjegaonkar, S.M.; Shanmugarajan, T.S. Methyl Jasmonate a Stress Phytohormone Attenuates LPS Induced in Vivo and in Vitro Arthritis. Mol. Biol. Rep. 2019, 46, 647–656. [Google Scholar] [CrossRef]
- Goel, Y.; Yadav, S.; Pandey, S.K.; Temre, M.K.; Maurya, B.N.; Verma, A.; Kumar, A.; Singh, S.M. Tumor Decelerating and Chemo-Potentiating Action of Methyl Jasmonate on a T Cell Lymphoma In Vivo: Role of Altered Regulation of Metabolism, Cell Survival, Drug Resistance, and Intratumoral Blood Flow. Front. Oncol. 2021, 11, 619351. [Google Scholar] [CrossRef] [PubMed]
| Activity | Plant | Action of Jasmonates | Literature |
|---|---|---|---|
| Germination | Pinus nigra Prunus armeniaca | inhibits pollen germination | [46,47] |
| Plant growth | Hordeum vulgare Avena sativa Triticum aestivum Helianthus annuus | inhibits the elongation of seedlings limits elongation of roots and stems | [48,49,50,51,52] |
| Ripening fruit | Fragaria chiloensis | prevents softening of the fruit | [53] |
| Aging of leaves | Oryza sativa Hordeum vulgare | accelerates the breakdown of photosynthetic pigments | [54,55] |
| Organ prolapse | Arabidopsis Rubisco | accelerates the fall off of pods and leaves | [56] |
| Male organ fertility in higher plants | Arabidopsis thaliana | reduces male fertility | [57] |
| Overall development | Medicago sativa | lengthens shoots slows down the induction of the embryo somatic differentiation process | [58] |
| Metabolic processes | Avena sativum Solanum lycopersicum | low concentration-stimulates the development of mycorrhiza high concentration-reduces the mycorrhization capacity of the roots | [42,43] |
| Formation of tubers and roots | Arabidopsis thaliana | increases the weight of tubers induces the formation of lateral roots | [59] |
| Jasmonates Derivatives | Cells | Concentration/IC50 | References |
|---|---|---|---|
| Methyl jasmonate | RAW264.7 (macrophage) | 50 and 100 μM | [65] |
| methyl 4,5-didehydrojasmonate DHJM | RAW264.7 (macrophage) | 6.25, 12.5, 25, and 50 μM | [68] |
| methyl 5,7,9,10-tetrabromojasmonate | melanoma cells B16-F10 | 0.042 mM | [69] |
| methyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) | 12.5 and 20 µM | [65] |
| t-butyl 5-chloro-4,5-didehydrodihydro-jasmonate | RAW264.7 (macrophage) | 3.12, 6.25, 12.5 and 25 μM | [67] |
| 3-((3-methyl-1,2,4-oxadiazol-5-yl) methyl)-2-(pent-2-en-1-yl)cyclo-pentanol | A549 SKOV-3 | 4564 mM 6077 mM | [70] |
| MJ-Mechanism of Anticancer Action | Cancer Cells | MJ IC50 | Literature |
|---|---|---|---|
| lymphoma B | 2 mM | [72] | |
| bioenergy involving ATP depletion via mitochondrial disturbance | mouse colon cancer CT-26 | 3 mM (max conc) | [73] |
| human T-lymphoblastic leucemia cell line MOLT-4 | 3 mM (max conc) | [73] | |
| mouse leucemia BCL1 | 3 mM (max conc) | [73] | |
| mouse melanoma B16 | 2.6 mM | [69] | |
| hepatocellular carcinoma HCC (LM3, BEL-7402, Hep3B, SMMC-7721) | 1.65 mM | [5] | |
| neuroblastoma SH-SY5Y | 3 mM (max conc) | [74] | |
| liver cancer Hep3B | 0.5 mM | [75] | |
| induction of re-differentiation by activation of the MAPK kinase cascade | human T-lymphoblastic leucemia cell line MOLT-4 | 0.5 mM | [75] |
| lung cancer A549 | 4.937 mM | [76] | |
| human breast cancer MCF-7 | 2 mM | [77] | |
| human melanocytic MDA-MB-435 | 1.9 mM | [78] | |
| leukemia HL-60 | 0.4 mM | [79] | |
| induction of apoptosis by the generation of ROS | glioblastoma C6 | 5 mM | [80] |
| non-small cell lung cancer A549 i H520 | 2 mM and 2.5 mM | [78] | |
| cervical carcinoma HeLa, CaSki, SiHa i C33A | 3.0 mM, 2.2 mM, 3.3 mM and 1.7 mM | [81] | |
| prostate cancer PC-3 | 5 mM | [82] |
| Jasmonates | Concentration/ Exposure Time | Organism | Effects | References |
|---|---|---|---|---|
| MJ | 0.5–3 mM, 24 h | C57BL/6 mice bearing EL-4 lymphoma | ↑ survival time compared to untreated control | [105] |
| MJ methyl 5,7,9,10-tetrabromojasmonate (synthetic) | 40 or 75 mg/kg body weight, 5 days a week, 3 weeks 20 mg/kg body weight, 5 days a week, 3 weeks | B16-F10 cells inoculated i.v. into the tail vein of C57BL mice to produce tumor growth in lungs | ↓ lung metastasis | [70] |
| MJ | 1–10 µM, 5 days | chorioallantoic (CAM) membrane of the chicken embryo | ↓ angiogenesis | [106] |
| MJ | 1 mg/1 mL, twice daily on the diseased skin or mucus for 4 weeks | pre-malignant and malignant skin lesions in 8 patients in the 56–73 age range | 3 patients showed positive responses 2 patients recovered 1 patient relapse three months after treatment | [107] |
| MJ | 1000 mg/kg body weight, 5 days a week over the next 8 weeks, | multiple myeloma in mice | overall survival for 150 days after MM cell injection | [108] |
| MJ | 20 and 40 mg/kg body weight | Wistar rats | ↓ lipopolysaccharide induced arthritis in rats | [107] |
| MJ | intra-tumoral administration | murine thymus-derived tumor Dalton’s Lymphoma | ↓tumor growth ↑survival of the tumor-bearing mice | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarocka-Karpowicz, I.; Markowska, A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. Int. J. Mol. Sci. 2021, 22, 8437. https://doi.org/10.3390/ijms22168437
Jarocka-Karpowicz I, Markowska A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. International Journal of Molecular Sciences. 2021; 22(16):8437. https://doi.org/10.3390/ijms22168437
Chicago/Turabian StyleJarocka-Karpowicz, Iwona, and Agnieszka Markowska. 2021. "Therapeutic Potential of Jasmonic Acid and Its Derivatives" International Journal of Molecular Sciences 22, no. 16: 8437. https://doi.org/10.3390/ijms22168437
APA StyleJarocka-Karpowicz, I., & Markowska, A. (2021). Therapeutic Potential of Jasmonic Acid and Its Derivatives. International Journal of Molecular Sciences, 22(16), 8437. https://doi.org/10.3390/ijms22168437






